Updated: 2023
Why enrich uranium?
- Natural uranium deposits exist all over the world, but uranium in this form is not suitable for nuclear weapons, and cannot be used in most nuclear reactors for either electricity or plutonium production.
- Natural uranium is composed of various isotopes, or different types of uranium. It contains approximately 99.3% of the isotope uranium-238, and has only very small concentrations, about 0.7%, of the fissile isotope uranium-235. An isotope is considered fissile if it can be split by a slow moving neutron.
- Uranium-235 is the most significant fissile isotope of uranium for reactor fuel and nuclear weapons. To be useful for either of these purposes, the concentration of uranium-235 must be increased by separating it from uranium-238 through a process known as enrichment.
Understanding Enrichment
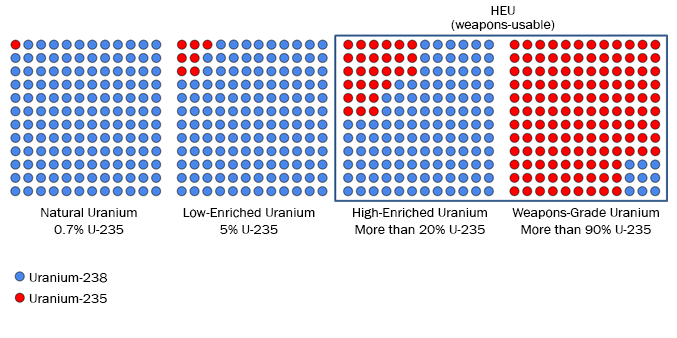
How much enrichment is necessary for use in reactors or weapons?
- Uranium enriched to concentrations above 0.7% but less than 20% uranium-235 is defined as low enriched uranium (LEU). Most civil and commercial nuclear reactors use LEU that is about 3-5% uranium-235.
- Uranium enriched to more than 20% uranium-235 is defined as highly enriched uranium (HEU). All HEU is weapons-usable, but the lower the enrichment level the greater the amount of material required to achieve a critical mass—the amount of material required to build a bomb.
- States with nuclear weapons typically use so-called weapons-grade HEU, which is typically defined as 90% HEU or above, to minimize weapons’ size. Smaller and lighter nuclear weapons are much easier to deliver; ballistic missiles in particular can only deliver highly miniaturized nuclear weapons.
- The graphic below is intended to help you to visualize both the mass and diameter of a bare spherical critical mass of enriched uranium (in a weapon design using a reflector), for various levels of enrichment. [1] As you can see in comparing mass and diameter for each critical mass, uranium has a similar density to gold and occupies much less volume for its weight than another metal like iron would.
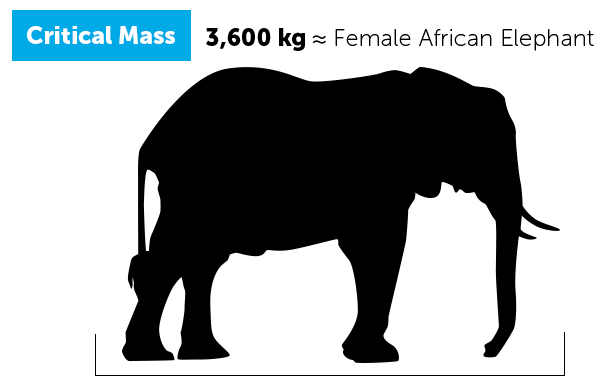
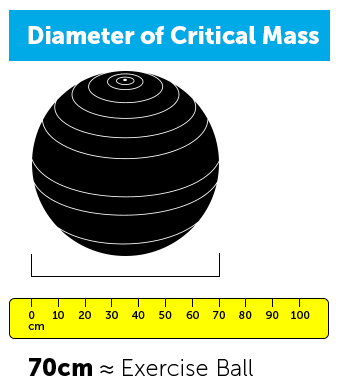
Different levels of enriched uranium are used for different purposes, and some levels are more suitable for use in nuclear weapons.
Higher concentrations of U-235 are more risky because you’d need less of it to build a bomb.
How is uranium enriched?
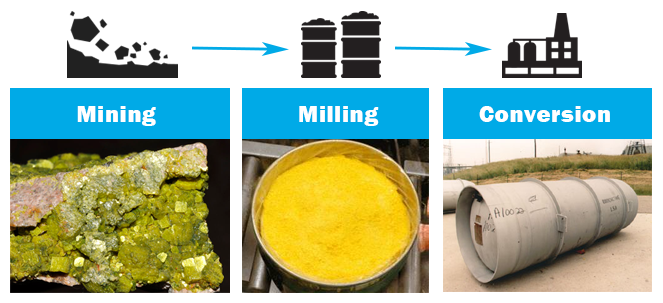
- Before uranium can be enriched, it must be mined from the ground, milled, and chemically processed.
- Natural uranium ore is mined from deposits in the Earth’s crust. Although uranium deposits exist all over the world, five countries possess 65% of the known supply of uranium ore: Australia, Kazakhstan, Russia, Canada, and Niger.
- The mined uranium ore is crushed to separate the uranium from the surrounding rock. This recovery process is known as milling, and produces uranium oxide concentrate (U3O8), commonly referred to as yellowcake.
- Yellowcake is transported to a conversion facility, where impurities are removed and the uranium is combined with fluorine to form uranium hexafluoride (UF6), a gas suitable for enrichment.
- Because uranium-235 and uranium-238 are chemically identical, chemical techniques normally used to purify substances cannot be used to separate them. Enrichment methods exploit the tiny difference in mass (about 1%) between the heavier, more abundant isotope uranium-238 and the lighter, fissile isotope uranium-235.
- Several methods have been used to enrich uranium. Electromagnetic isotope separation (EMIS) was employed by the United States during the Manhattan Project, but it required immense amounts of electricity and was abandoned after the war because of its poor efficiency and high costs. Throughout the Cold War, gaseous diffusion was the dominant enrichment technology, but it also required immense amounts of electricity and large facilities. Laser excitation is a relatively new enrichment technology that has not yet proven commercially viable.
- Today, the most common and efficient enrichment method uses gas centrifuges. To learn how a gas centrifuge works, watch the video below and click through the 3D graphic.
Why does enrichment technology pose a proliferation risk?
- Uranium enrichment poses a nuclear proliferation risk because the same technology that can produce LEU for reactor fuel can also be used to produce HEU for nuclear weapons. There are no technical barriers to prevent countries with enrichment capabilities from using them to enrich uranium to the higher levels required for nuclear weapons, only legal prohibitions.
- The Treaty on the Non-Proliferation of Nuclear Weapons (NPT) prohibits non-nuclear weapon states (NNWS) from developing or acquiring nuclear weapons, and commits the five treaty-recognized nuclear-weapon states to pursuing nuclear disarmament. The NPT also requires NNWS to negotiate and conclude safeguards agreements with the International Atomic Energy Agency (IAEA), whose inspectors are tasked with monitoring states’ programs to verify that their declarations about their nuclear materials and activities are correct and complete. (See NPT Tutorial)
- The NPT does not expressly prohibit non-nuclear weapon states from possessing enrichment facilities, as long as these facilities are used for peaceful purposes, declared, and placed under IAEA safeguards. Covert enrichment facilities, which have not been declared by a state to the IAEA, are a serious proliferation concern.
- EMIS and gaseous diffusion are not ideal technologies for covert proliferators, as they are easily detected using satellite imagery analysis and other intelligence tools because of their large infrastructure requirements. Nonetheless, Iraq successfully hid an EMIS-based enrichment program from the world in the 1980s, perhaps in part because the U.S. and other intelligence communities assumed proliferating countries would not consider EMIS.
- Centrifuges pose a unique proliferation challenge because detecting covert facilities in a timely manner is very difficult and existing centrifuges can be quickly reconfigured to produce HEU.
- Currently, France, the United Kingdom, the Netherlands, Germany, the United States, Russia, Argentina, Brazil, India, Pakistan, and Iran are known to have uranium enrichment capabilities. [2]
- Breakout time is the theoretical amount of time required for a country to reconfigure an existing enrichment plant to produce enough HEU for a nuclear weapon. Breakout time only becomes relevant if a country makes the political decision to pursue nuclear weapons. While breakout time is regularly used in news reports about nonproliferation, states attempting to create a nuclear weapon are unlikely to base any real policy on breakout time calculations.
- Breakout time is less when starting from an existing stockpile of uranium enriched to 3-5% uranium-235—the level used for nuclear power—than when starting with natural uranium. Enrichment removes unwanted uranium-238, making the concentration of uranium-235 atoms higher. It takes much more work to enrich uranium to 3-5% uranium-235 (typical power reactor fuel), than it does to further enrich uranium from 3-5% to 90% uranium-235 (weapons-grade material).
- Starting out with natural uranium, a facility with nearly 6,000 early-generation centrifuges could produce about 40 kg of weapons-grade uranium within a year. [3]
- If material pre-enriched to 3.5% uranium-235 is used, a facility with the same capacity could produce about 180 kg of weapons-grade uranium per year. [4]
- Breakout time calculations do not take into account the other steps involved in acquiring nuclear weapons, such as designing, fabricating, testing, and deploying nuclear weapons on delivery systems. Although these steps could be pursued in parallel with the production of fissile material, they should be considered when assessing a country’s breakout potential.
- A country could also “sneak out” by covertly constructing a small undeclared facility dedicated to the production of HEU to avoid IAEA safeguards altogether. A covert centrifuge plant could be small, underground, and therefore very difficult to detect remotely. [5]

Inspection of Iraq’s EMIS Program, Source: www.llnl.gov
What are the chances that a country attempting to either “breakout” or “sneak out” would be discovered?
- History shows countries are far more likely to “sneak out” than “breakout” precisely because breaking out is not covert. If a non-nuclear weapon state party to the NPT breaks out using a declared facility safeguarded by the IAEA, international inspectors would almost certainly detect the production of weapons-grade HEU or the diversion of LEU for further enrichment elsewhere. Although the time between detection and production of enough material for a nuclear weapon might be short, particularly if centrifuges are used, the proliferating country would still risk international pressure, including economic sanctions and possible military action.
- Sneaking out might not be detected by the international community in a timely manner. Although a number of covert nuclear facilities have been revealed in recent years through intelligence sources and methods, some programs have remained undetected longer than others. All six countries found to have violated their NPT obligations with illicit nuclear activities did so by maintaining covert facilities (Iran, Iraq, North Korea, Libya, Romania, and Syria). [6]
Sources:
[1] See Table 1: Alexander Glaser, “On the Proliferation Potential of Uranium Fuel for Research Reactors at Various Enrichment Levels,”Science and Global Security, Vol. 14, 2006, pp. 1-24, http://www.princeton.edu/~aglaser/2006aglaser_sgsvol14.pdf.
[2] “Uranium Enrichment,” World Nuclear Association, October 2022. https://world-nuclear.org/information-library/nuclear-fuel-cycle/conversion-enrichment-and-fabrication/uranium-enrichment.aspx.
[3] Houston G. Wood, Alexander Glaser, and R. Scott Kemp, “The gas centrifuge and nuclear weapons proliferation,” Physics Today, (September 2008), p. 45.
[4] Houston G. Wood, Alexander Glaser, and R. Scott Kemp, “The gas centrifuge and nuclear weapons proliferation,” Physics Today, (September 2008), p. 45.
[5] R. Scott Kemp, “The Nonproliferation Emperor Has No Clothes: The Gas Centrifuge, Supply-Side Controls, and the Future of Nuclear Proliferation,” International Security, Vol. 38, No. 4 (Spring 2014), p. 48-50.
[6] James M. Acton, “Who Cares about an Iranian Nuclear Breakout? Beware of an Atomic ‘Sneak-out,'” The National Interest, (November 4, 2014), www.nationalinterest.org.
Photo Credits for the “How is Uranium Enriched” Graphic
[1] Uranium Ore, Parent Géry, WikiMedia Commons
[2] Low Enriched Uranium, U.S. Government, WikiMedia Commons
[3] UF6 container Type 48Y, Nuclear Security & Safeguards Education Portal, G. Eccleston E. Wonder, nsspi.tamu.edu, accessed September 8, 2015